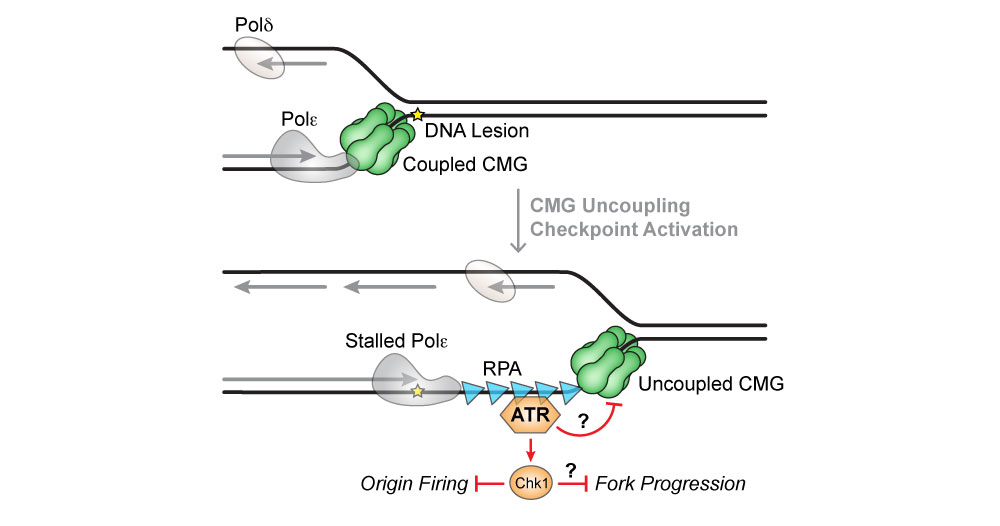
Replication stress refers to conditions that slow down or stall replication. Replication stress often causes the CMG helicase to uncouple from DNA synthesis, leading to a buildup of single-stranded DNA (ssDNA) that can cause DNA breaks and genome instability. ssDNA generated by the uncoupled helicase activates the DNA damage checkpoint tasked with detecting and responding to RS. We are using the KEHRMIT single-molecule platform to study the interplay between helicase function and checkpoint signaling.
The eukaryotic replisome is a large molecular machine comprised of 60+ individual subunits, including the replicative helicase, the replicative primase-polymerase, the leading and lagging strand replicative polymerases, various processivity factors, DNA repair factors, regulatory subunits, structural scaffolds, etc. Although several subunits are thought to readily bind/dissociate from this complex, very little is known about the dynamics of subunit exchange and how it is tuned to regulate the function of the replisome as a whole. To tackle this problem we are imaging the exchange of fluorescently-labeled replisome subunits in Xenopus egg extracts.
In addition to employing the workhorse KEHRMIT assay, we are actively developing new tools and reagents to study genome maintenance mechanisms at the single-molecule level.